Seminar Report : Safety Assessment for New Drugs using Real World Data
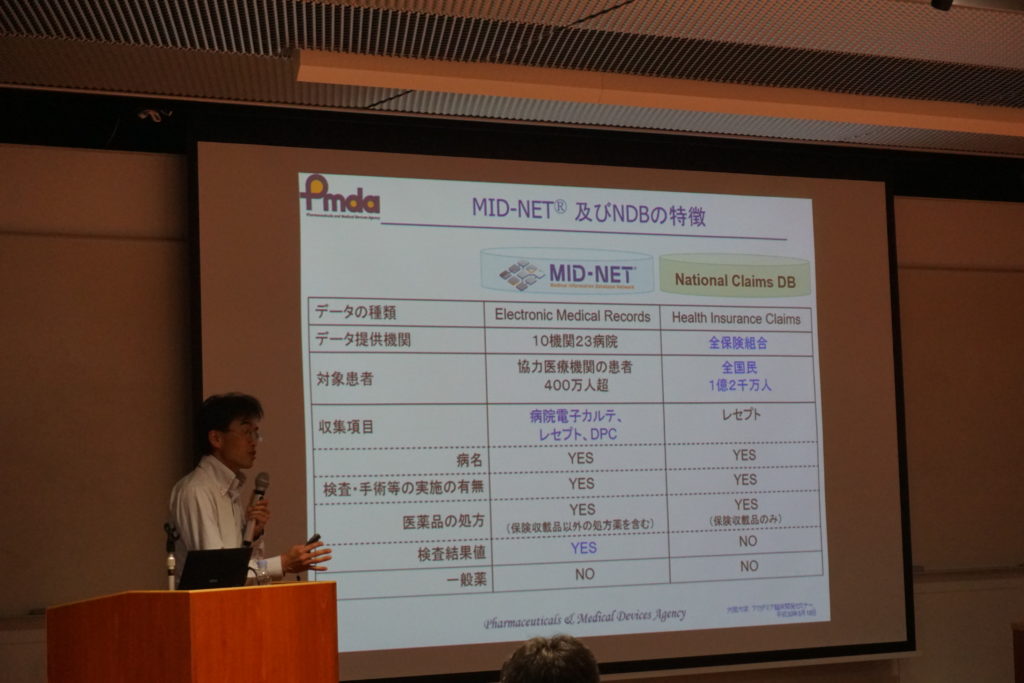
On Friday May 18th, the 1st Academia Clinical Development Seminar in 2018 fiscal year was held at the multi-media hall, Osaka University Hospital. We invited Dr. Yoshiaki UYAMA as a lecturer from the Pharmaceuticals & Medical Devices Agency (PMDA) with a title of “safety assessment for new drugs using real world data”. He previously gave us a lecture about “ICH-E17 guideline” last December so that this was his second lecture in our hospital. He introduced a new medical database system called “MID-NET®” launched since April 2018, with his experience of great efforts for development and the purpose and usage instruction of this database.

At the beginning of his talk, he emphasized the importance of recognizing the limit of pre-approval data on drug safety. (Generally speaking,) each clinical trial included, at most, hundreds to thousands of cases treated with a new investigational drug, leading to missing (overlooking) adverse events in the experimental (development) stage. On the other hand, the data would be obtained from huge samples after the approval and could allow us to detect those (missed) adverse events.

His team has launched “MIHARI project” as safety monitoring of drugs under collaboration with 23 hospitals nationwide in Japan and they collected clinical information on laboratory data and medical prescriptions from electrical charts in each hospital through secured ICT. At the initiating of the project, there were a bunch of troubles combining data, because laboratory data and treatment strategies were not standardized among these hospitals. They accomplished standardization of each variable with reliability through their greatest efforts. He gave us typical examples that had revised precautions in drug package inserts based on their analyses of the data.
He mentioned that academia and pharmaceutical companies could use MID-NET® ( for a fee). He would like us to use it as safety monitoring with more scientific, more effective, and more epidemiological in researches. Besides, his team started doing research and development of drugs with a usage of the database called “real world data” or “big-data”, because we can obtain not only safety, but also efficacy data. He told that academia could use the database proactively and then his talk was ended with big-claps.
We are really interested in real world- and big- data because we are aiming to get approval for pharmaceutical laws for new drugs or medical devices through clinical studies. More than 50 participants attended the lecture. They must have felt that his talk was very advanced and impressive. After the lecture, there were a lot of questions from the participants. We were satisfied with his comments that he would like to collect information on comprehensive medical data through the infrastructure.

The 2nd Academia Clinical Development Seminar will be held on Friday June 15th, starting from 5:30pm to 7:00pm. The lecturer will be Prof. Kou NAKATA from Niigata University Medical and Dental Hospital under the title of “Light and shadow of the development of new drugs for rare diseases –to overcome the Darwinian Sea-. Don’t miss it!


