We held the 3rd Global Clinical Trial Advanced and Planning Symposium under the theme of “Glowing platform of global clinical research from competition to collaboration”.

On Thursday 7th Mach 2019, we held the 3rd Global Clinical Trial Advanced and Planning Symposium under the theme of “Glowing platform of global clinical research from competition to collaboration”.
This symposium was jointly organized by Osaka University Hospital and National Cancer Center Hospital, and supported by Japan Agency for Medical Research and Development (AMED) as a part of Japan Agency for Medical Research and Development, Translational Research Program; Strategic Promotion for Practical Application of Innovation Medical Technology (Global Clinical Trial Development Project).
In order to boost and enhance R&D and expand our seeds from Japanese academia to overseas, we decided the theme of the 3rd symposium as " Glowing platform of global clinical research from competition to collaboration” in hope that clinical research core hospitals in law advance to the next phase in which they unite and work together in promoting further R&D, rather than being isolated and making individual efforts as they have been doing.
As you may know, basic research in Japan has been recognized as superior among countries, but clinical research in Japan has not. To overcome this situation, we thought we need to collaborate each other across Japan to promote R&D further especially in clinical part.
This symposium consisted of 5 sessions, because what we have come to realize that “Working in Collaboration” is the most important task for us in order to promote the best outcomes in implementation, planning, designing and supporting of MRCT. The 5 sessions included: 1) Government, Industry-Academic Approaches, 2) Consortium Activities, 3) Various Projects related to MRCT, 4) Usage of Real-World Data (RWD) and anticipated challenges in research, and 5) Translational Research and Early Exploratory Clinical Trials in Asia.
Part 1-Session 1: Office Director, Junko SATO from Pharmaceutical and Medical Devices Agency (PMDA), Professor Hiroki NAKATANI from Keiko University and Group Manager, Mr. Ryohei IWASAKI from Novartis Pharma K.K gave us their talk regarding expectations and issues from their own perspectives on “Industry-Academia-Government Collaboration Approach to Promote Globalization of Clinical Research”.

As domestic representative facilities, “Research Studio powered by SPARK” lead by Dr. Tomoyoshi KOYANAGI from University of Tsukuba and then Dr. Daisaku NAKTANI from Osaka University Hospital introduced their efforts on Japan Consortium of Clinical Research Core Hospital (J-CCRC) respectively.

The afternoon session followed by the morning session, “Leveraging Real-World Data (RWD): Challenges and Opportunities with use of RWD” ran in parallel with J-CCRC meeting. The presentation of Mr. Ryuji TOKIWAGI from Ebiya Ltd. was one of the interesting and curious presentation, because it could beg the question us how to use or relevant to the medicine. After that, Dr. Taro SHIBATA, National Cancer Center lectured clinical research with RWD from the view point of biostatistician.

In response to the inquiries concerning actual projects from last year's symposium questionnaire, in the session 4 “Introduction of Global Clinical Research Projects", Dr. Yuji KUMAGAI from Kitasato University Hospital, Dr. Mitsumi TERADA from European Organization for Research and Treatment of Cancer, and Dr. Yasutoshi KIDO from Osaka City University, Dr. Yoshikazu YONEMITSU from Kyushu University talked about their current projects in progress with numerous photos.

Lastly, in the session of “Future Prospects for Global Clinical Research from the Perspective of Academia”, we welcomed three more talks: Dr. Jun YOSHIDA from Ministry of Health, Labor and Welfare, Professor Kyung-Sang YU from Seoul National University, Korea, and Dr.Terra DAI from Shanghai Clinical Research Center, China. They addressed lectures in relation with translational research and efforts of early exploratory clinical trials.

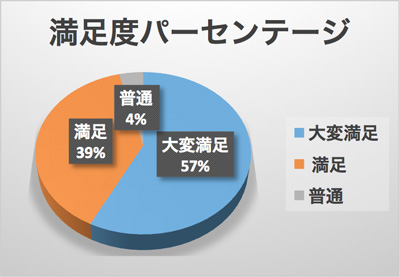
Gratefully, more than 190 people registered for the symposium this time from various field, including universities, university hospitals, other hospitals, research institutes, pharmaceutical companies, and public offices. According to the questionnaire survey after the symposium, thankfully little over 90% of participants were satisfied and would like to participate the next one.
Followings are some positive opinions and comments received:
“It was a great time to get know how much doctors are enthusiastic toward Investigator-initiated (clinical) trial: IIT”.
“The symposium was interesting to hear the story and experience about enthusiasm, research difficulties, more challenges to face and ways.”
“It was helpful to hear about the future direction of MRCT of the Ministry of Health, Labor and Welfare.”
“We would like to know more case studies for further knowledge”
Following the great success in the 2nd symposium that was held last year, we would like to thank all the participants and parties related to the 3rd symposium.
We look forward to seeing you again when the 4th Global Clinical Research Support Symposium will be held, and we will do our best to prepare the valuable program to further progress global clinical research by taking advantage of the past three experiences.
Notably, in conjunction with this symposium, “The 4th Global Clinical Trial Development Project Japan Consortium of Clinical Research Core Hospital (J-CCRC)” meeting was held in parallel. We shared the information on current efforts toward MRCT amongst 12 clinical core hospitals across Japan, and continued to discuss regulatory situation of pharmaceuticals and insurance in Asian countries.
The next 5th J-CCRC will be scheduled in September 2019. We would like to continue working to make another great, meaningful and fruitful meeting.

【Reference】
- ・First Global Clinical Trial Advanced and Planning Symposium
- ・Second Global Clinical Trial Advanced and Planning Symposium in Osaka.
Thank you very much for the cooperation of chairpersons
- Dr. Daisuke SUGIYAMA from Kyushu University
- Dr. Tatsuya MARUYAMA from Keio University
- Dr. Kenichi NAMAKMURA from National Cancer Center Hospital
- Dr. Tadao TAKANO from Tohoku University Hospital
- Dr. Shinobu SHIMIZU from Nagoya University Hospital
- Director, National Cancer Center Hospital
- Osaka University: Akira MYOUI Tomomi YAMADA, Eisuke HIDA


